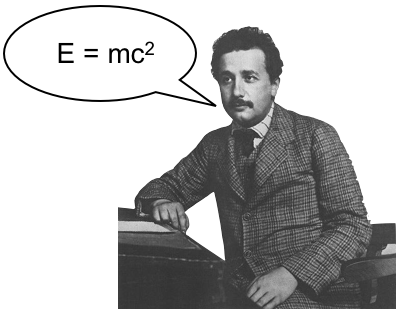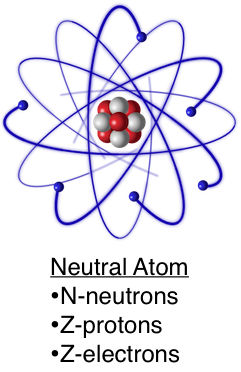
An atom is composed of three types of particles: "Z" positively charged protons, "Z" negatively charged electrons, and "N" neutrons, which have no charge. The atomic number of an atom is the sum of the number of protons and neutrons in the nucleus.
The protons and neutrons are tightly bound together by the strong nuclear force and form the nucleus of the atom. As the neutrons have no electrical charge, the nucleus is positively charged. Electrons, being negatively charged, are bound to the nucleus by the Coulomb force. An atom has an equal number of protons and electrons, and is electrically neutral. By changing the number of electrons, an ion is created. Ions are not electrically neutral and can therefore can be more easily manipulated with electric and magnetic fields for transport or trapping.
The protons and neutrons are tightly bound together by the strong nuclear force and form the nucleus of the atom. As the neutrons have no electrical charge, the nucleus is positively charged. Electrons, being negatively charged, are bound to the nucleus by the Coulomb force. An atom has an equal number of protons and electrons, and is electrically neutral. By changing the number of electrons, an ion is created. Ions are not electrically neutral and can therefore can be more easily manipulated with electric and magnetic fields for transport or trapping.
Naively one might think that the mass of an atom is simply given by the sum of the masses of its constituents (electrons, protons and neutrons). However, this is not the case, and can be described using the mass-energy equivalence relation. Some amount of the total energy of the system is required to bind it all together, called the binding energy. As the nucleus is very strongly bound, most of the binding energy is tied up there, but there is also binding energy associated with the bound electrons. The mass of an atom is then the sum of the masses of all the individual particles, minus the amount of energy holding them all together.